A Letter of Intent (LOI) has many definitions and uses in business and in other areas, but here we will focus on the LOI when it comes to funding biomedical research. The LOI is completed as the first of two steps required to submit an application to some non-dilutive funding mechanisms. We will utilize the Congressionally Directed Medical Research Programs (CDMRP) as an example. Many of the research programs within CDMRP utilize LOIs for program planning purposes to inform their reviewer recruitment. The LOIs are not reviewed during either the peer or programmatic review sessions, but the content narrative is important for identifying and recruiting topic- and focus area-matched subject matter experts/reviewers for the full application you submit.
LOI vs. Pre-Proposal
The CDMRP has two types of pre-applications: LOIs and pre-proposals. The LOI narrative should be a brief description of the project to be conducted. LOIs are reviewed administratively to assist with the two tiers of review. An invitation to submit a full application is not required following submission of an LOI.
Pre-proposals are more detailed pre-applications that should describe the proposed project using the guidelines provided in the appropriate program announcement. Pre-proposals are screened by the program-specific review committee. An invitation is required to submit a full application.
LOI Format and Content
For CDMRP, there is typically a one-page limit for the LOI. The LOI should be on the prime or lead submitting company’s, University’s, or Foundation’s letterhead and should be signed by the lead principal investigator (PI) at the end. “Dear Program Planning Committee” is a common salutation. Include the fiscal year (FY), research program acronym and mechanism Focus Area(s) under which the proposal/application will be submitted. Some suggestions for providing a brief description of the research to be conducted and other pertinent information is provided in the template below. Be sure to check the program announcement for the award mechanism for which you are applying to verify the required format and information for the LOI.

Pre-Application Submission Process
All pre-application components must be submitted by the PI through the Electronic Biomedical Research Application Portal (eBRAP; https://eBRAP.org/). During the pre-application process, eBRAP will assign each submission a unique log number. This unique eBRAP log number is required during the full proposal/application submission process.
The following information comes from the CDMRP General Application Instructions, Version 20190530. It is important to ensure that you are following the most recent General Application Instructions which accompany the Program Announcements released for each funding opportunity.
There are six tabs to be completed for the pre-application to be submitted through eBRAP. The LOI letter will be uploaded as Tab 5 of the pre-application package and has a one-page limit.
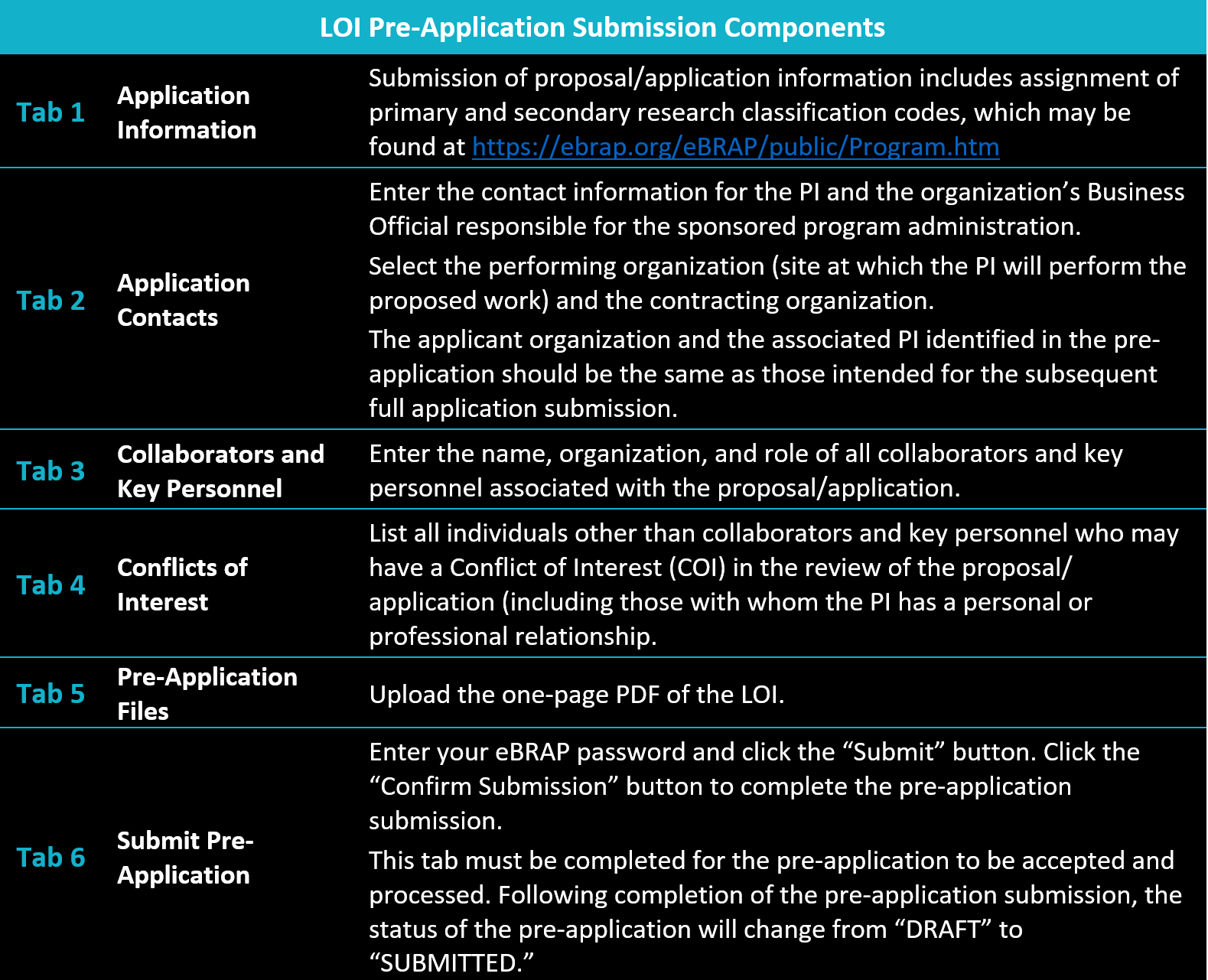
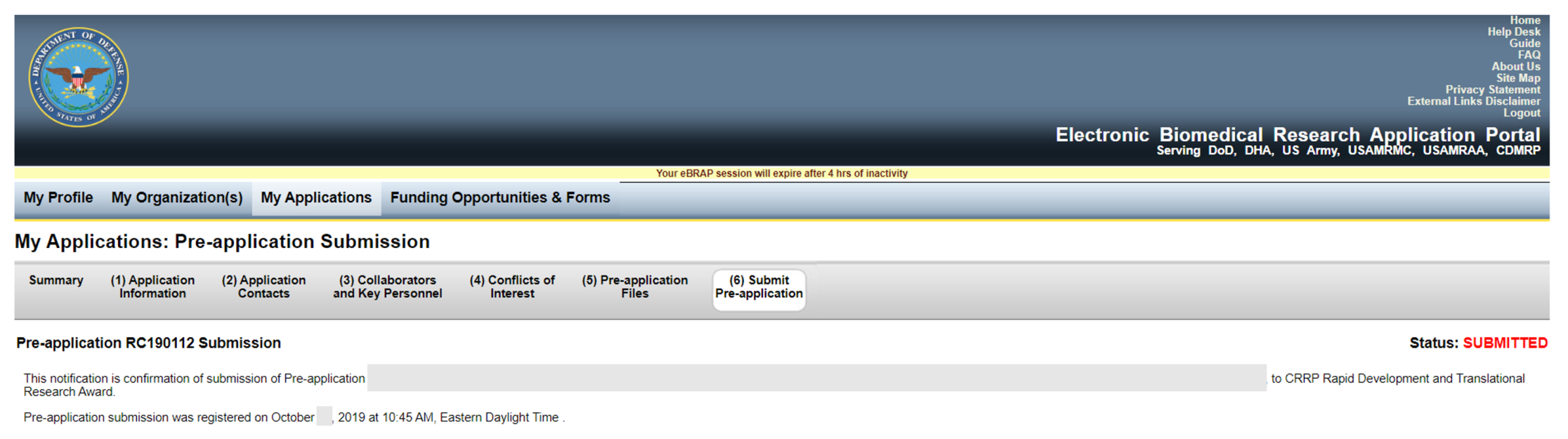
When the pre-application has been submitted you will receive a confirmation of pre-application submission registration in eBRAP similar to the screenshot below.
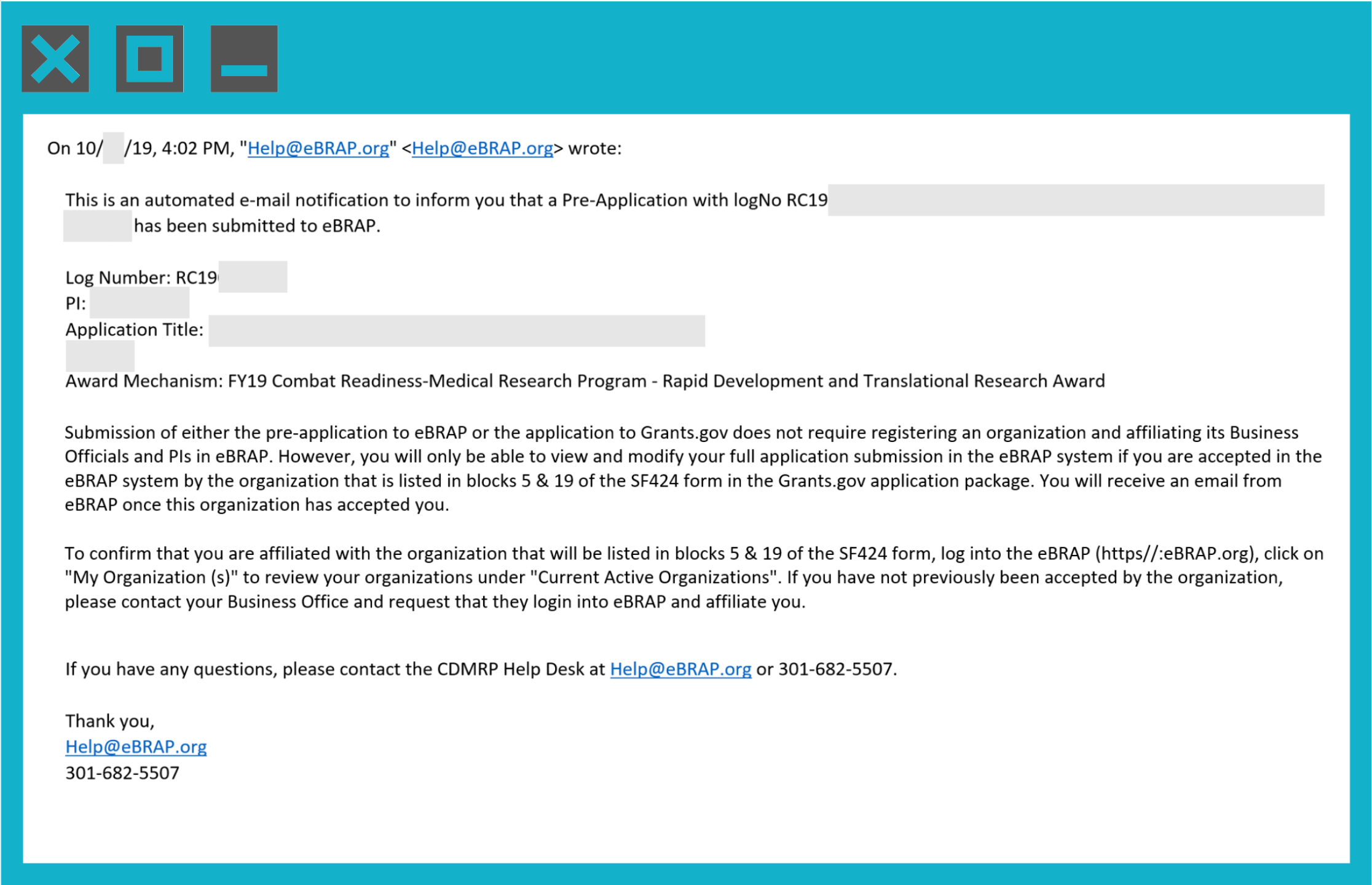
The lead PI will also receive an email confirmation that will look like the following example below:
Can you make changes to the pre-application LOI?
Up until the deadline submission, which is typically listed by date and Eastern time zone, you can edit the tabs and remove an uploaded file to replace it with an updated version. If any changes are necessary after submission of the pre-application (LOI), the PI can contact the CDMRP Help Desk at help@eBRAP.org or 301-682-5507. We have always found the customer service personnel to be very helpful.
What’s Next
LOIs in the pre-application submission process are the least labor intensive first step of a two step application submission process. Understanding the information and steps required ahead of the submission deadline will save unnecessary work required to complete this requirement. If you would like to add information to this article, please share in the comments below. I hope that you are successful in all of your non-dilutive funding pursuits.
